Implementing Genomic Medicine in Pediatric Cancers: Department of Pediatric Oncology, National Cancer Center Initiative
Chitose Ogawa
2023/12/15
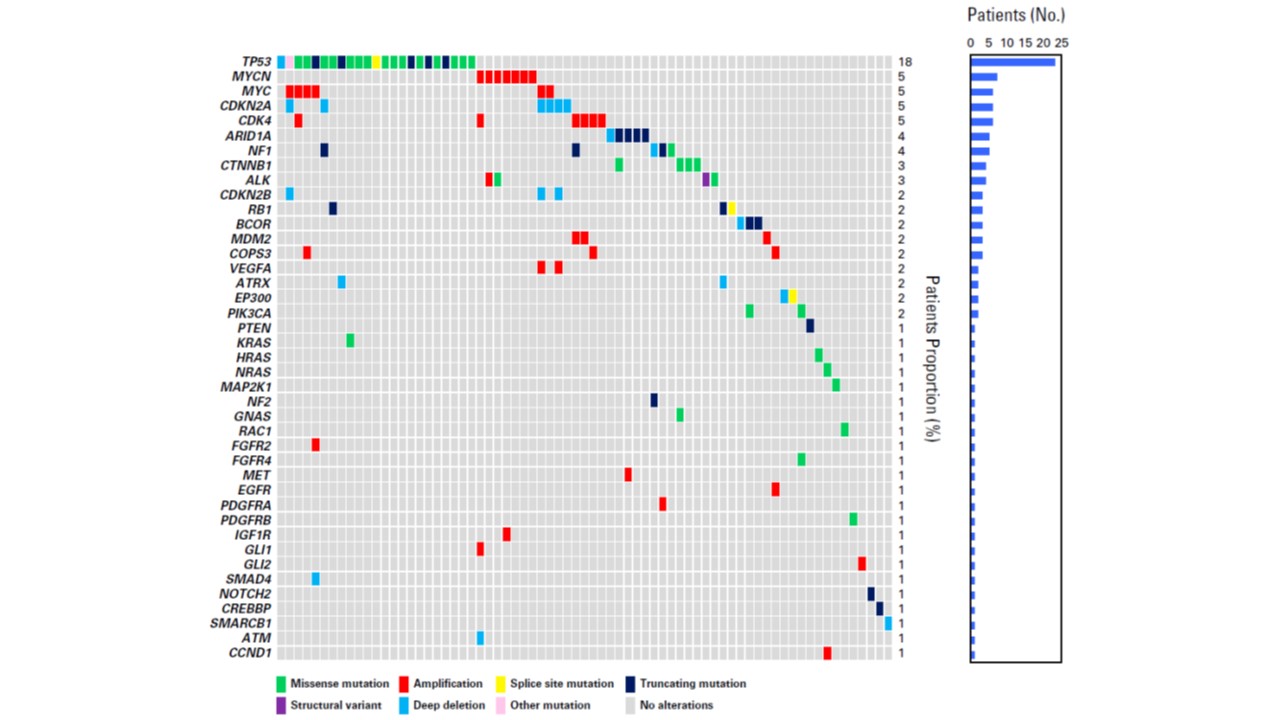
The official approval of cancer gene panel testing has enabled us to look for potentially effective therapeutic options for patients with pediatric cancers. In particular, tumors harboring such as NTRK or ALK gene fusion can shrink or disappear in the image with the use of TRK or ALK inhibitors, respectively. Through participating and initiating international clinical trials and research projects, we are striving to provide optimal treatment for pediatric cancers and to deliver medically necessary medications to the patients who need them for their lives.



