Efficacy of Anti-HER2 Therapy with Trastuzumab Deluxecan in Uterine Carcinosarcoma was confirmed.
Tatsunori Shimoi
2023/6/1
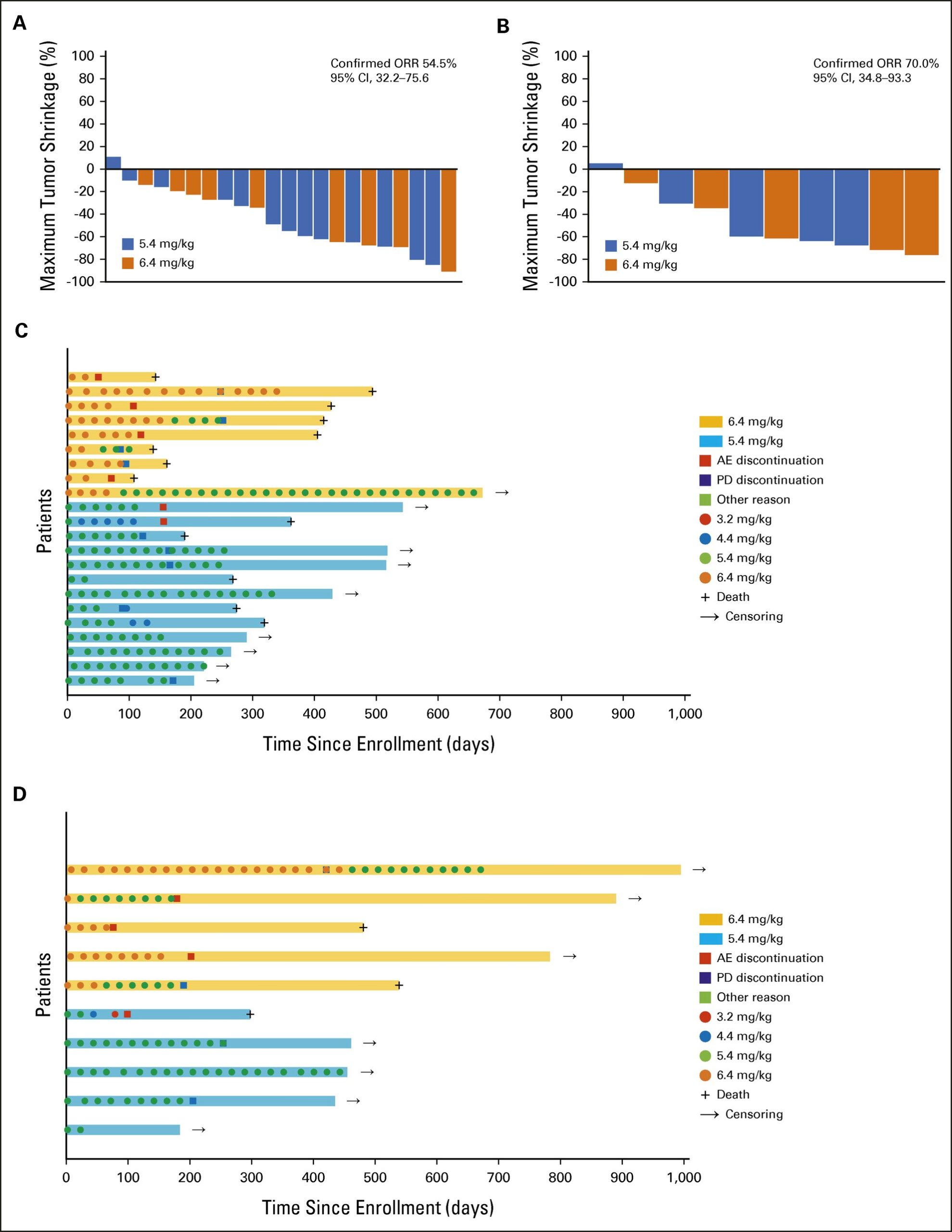
In recent years, there have been remarkable advancements in cancer drug therapy, leading to the development of novel drugs that are effective in treating rare cancers. The MASTER KEY project, a multicenter collaborative study conducted at the National Cancer Center Hospital, comprises a prospective registry study and several clinical trials aimed at addressing advanced rare cancers, cancers of unknown primary origin, and rare tissue subtypes of common cancers. Our STATICE trial, one of the clinical trials conducted under this project, is a phase II investigator-initiated clinical trial that evaluated the efficacy of the anti-HER2 antibody-drug conjugate trastuzumab deruxtecan in patients with uterine carcinosarcoma who have HER2, a protein that plays a critical role in cancer cell growth, at seven sites in Japan. Our findings revealed that 54.5% of patients with high HER2 expression and 70% of patients with low HER2 expression exhibited at least a 30% reduction in tumor size (overall response rate). Our study, the first in the world to demonstrate the potential of anti-HER2 therapy in treating uterine cancer sarcoma, was published in the Journal of Clinical Oncology on March 28, 2023 US time. The results of our research suggest that trastuzumab deruxtecan may represent a novel treatment option for patients with uterine carcinosarcoma that expresses HER2 protein, and are expected to further accelerate the development of more effective treatments for rare cancers for which large-scale clinical trials are difficult to conduct.



