HARMONY

- For patients and health care professionals
- For participating companies/list of participating companies
- List of researchers by participating facilities
Outline of the Project
The HARMONY study is part of the ATLAS project and is a collaborative study with institutions in Asia to study small residual cancers (microresidual lesions) that remain after radical treatment.
Patients with HER2-positive breast cancer who are scheduled for surgery after preoperative chemotherapy will have blood samples taken periodically to test for circulating tumor DNA (ctDNA) in the blood.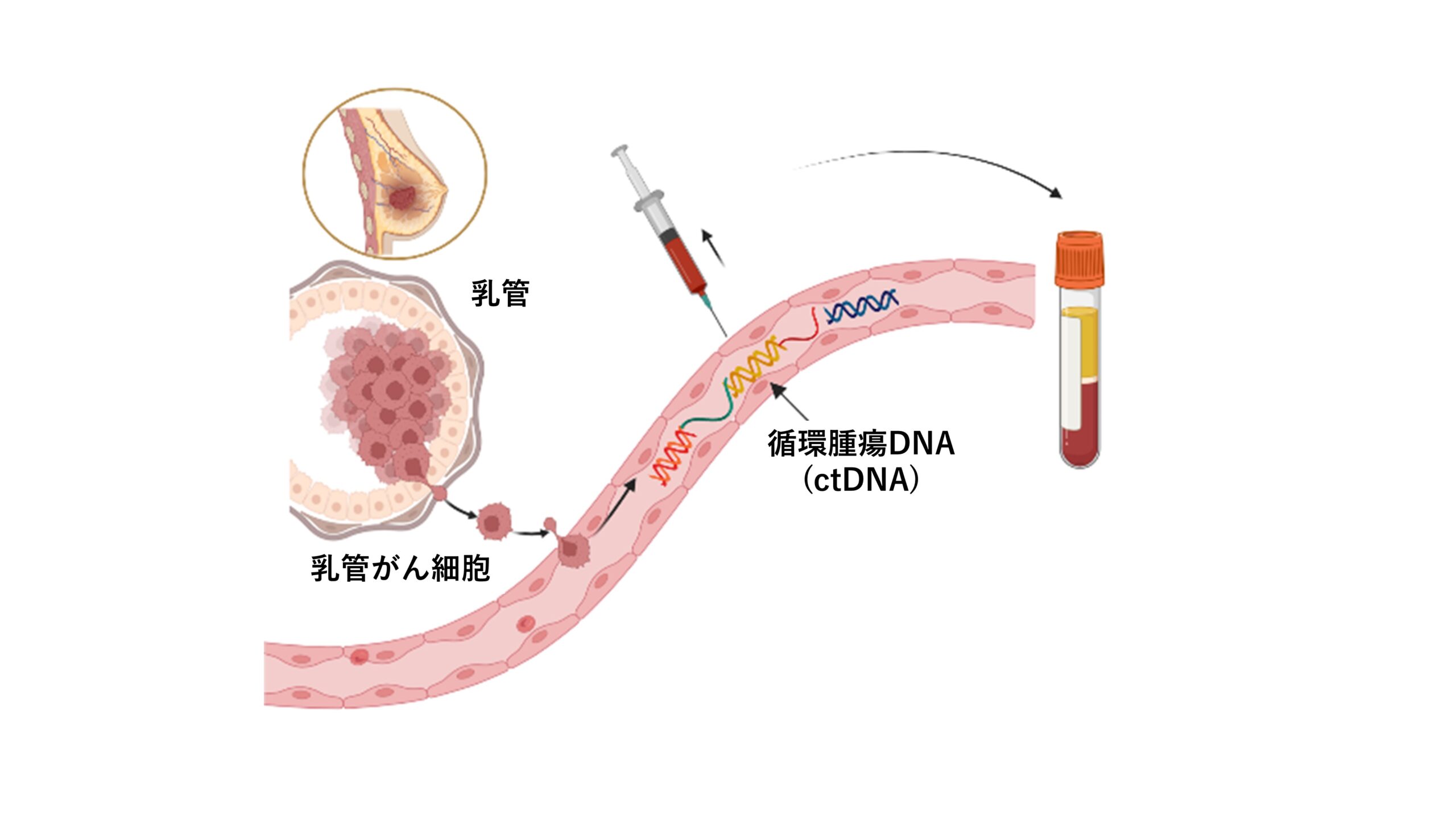
The method of using a patient’s blood, urine, or other bodily fluids to test for cancer genomes is called liquid biopsy. This method is less invasive and provides results more quickly than conventional biopsy. Among them, the test using ctDNA is being studied for clinical application for early diagnosis of cancer, detection of small residual lesions, post-treatment monitoring, and detection of genetic abnormalities at the time of recurrence.
This study aims to investigate whether the presence or absence of ctDNA after curative treatment correlates with recurrence, as well as to analyze the genetic information of Asian breast cancer patients. If the usefulness of liquid biopsy is checked through this study, it will be possible in the future to provide personalized medicine, such as adding or omitting postoperative adjuvant therapy depending on the presence or absence of microscopic residual disease. It is also expected to predict recurrence at an early stage and provide treatment based on the genetic abnormality of the cancer.
Origin of the Project Name
This study is called “Asian multicenter prospective study in HER2 positive early breast cancer for detecting minimal residual disease by circulating tumor DNA analysis with neoadjuvant chemotherapy. The term “HARMONY” is an abbreviation for “Asian multicenter prospective study in HER2 positive early breast cancer for detecting minimal residual disease by circulating tumor DNA analysis with neoadjuvant chemotherapy.
The word “HARMONY” is intended to express our desire to harmonize with other Asian countries in order to promote joint research on cancer treatment.



